An alarming increase in mortality rate shadows acute kidney injury (AKI) cases in Indonesia.
Health Minister Budi Gunadi Sadikin has said that the death rate for acute kidney disorders was approaching 50 percent. As of Tuesday 18th, October 2022, 206 cases had been recorded.
Children under five who are exposed to this disease reach around 70 per month. He even said that the reality is certainly more than the available data.
At Cipto Mangunkusumo Hospital (RSCM) in Jakarta, which is a referral hospital, the mortality rate for patients with acute kidney disease is even more than 50 percent.
The Health Ministry also stated that children who had already consumed the five brands of syrup that were recently withdrawn by the Food and Drug Administration aren’t required to panic or go to the laboratory for kidney function tests, namely urea and creatinine.
The Head of the Communication and Public Service Bureau of the Health Ministry, Siti Nadia Tarmizi, said that the precautions that parents need to do is to monitor the frequency and amount of their child’s urine. If they have oliguria (not much urine) or anuria (no urine at all), they must be taken to a health facility.
“This is an acute disease, the important thing is to monitor the frequency and amount of urine. If you don’t take medicine, the kidneys can still filter according to its function,” said Tarmizi when contacted by CNNIndonesia.com on Friday 21st October 2022.
The results of the ethylene glycol (EG) contamination test in some syrup drugs have not been able to support the conclusion that the use of these drugs has a relationship with the incidence of atypical progressive acute kidney disorders in Indonesia.
There are still several risk factors causing this mysterious disease based on preliminary investigations, such as viral infection, Leptospira bacteria, a multisystem inflammatory syndrome in children (MIS-C), or post-COVID-19 multisystem inflammatory syndrome.
“The Health Ministry asks all pharmacies to temporarily not sell over-the-counter or limited-free drugs in liquid or syrup form to the public until the results of the search carried out by the Health Ministry and Food and Drug Administration is complete,” she said.
The Food and Drug Administration has previously determined five brands of syrups containing EG compounds that exceed the threshold after conducting tests on suspected compound contamination in 39 batches of 26 medicinal syrups up to 19th October 2022.
The reference used in the test is the Indonesian Pharmacopoeia and/or other references in accordance with law number 36 of 2009 concerning health as the national standard for quality assurance of all circulating drugs.
According to Pharmacopoeia and recognised national standards, the safe threshold or tolerable daily intake (TDI) for EG and DEG contamination is 0.5 mg/kg body weight per day. The following are the five syrups in question:
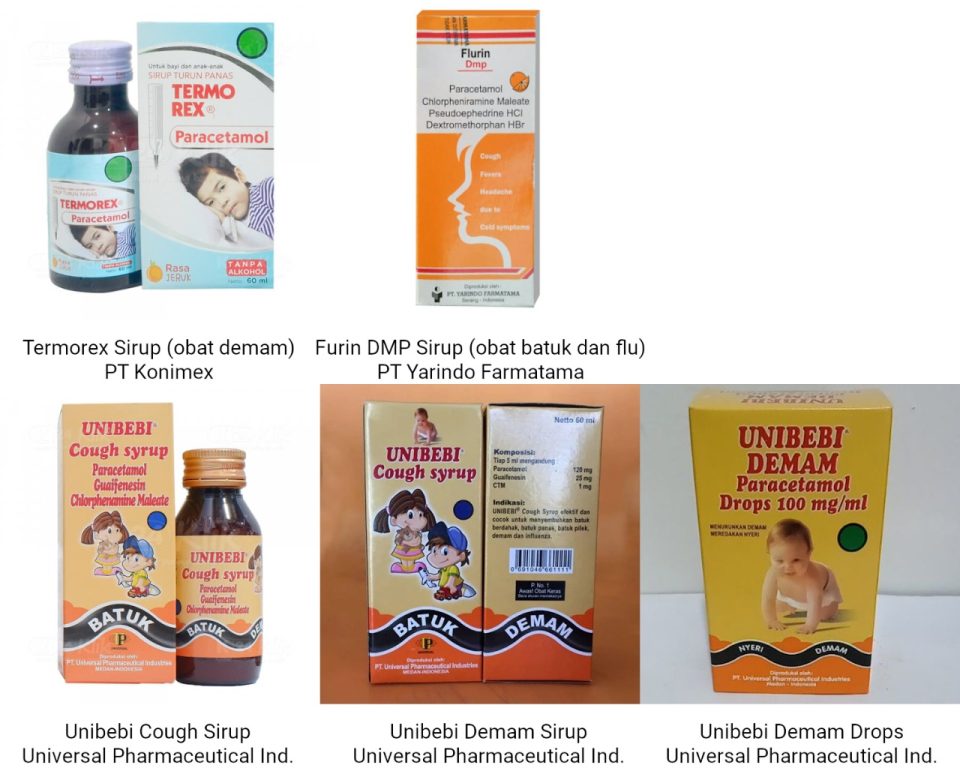
- Termorex Syrup (fever medicine), PT Konimex production with distribution permit number DBL7813003537A1, box packaging, plastic bottles @ 60 ml.
- Flurin DMP Syrup (cough and flu medicine), produced by PT Yarindo Farmatama with distribution permit number DTL0332708637A1, box packaging, plastic bottle @ 60 ml.
- Unibebi Cough Syrup (cough and flu medicine), produced by Universal Pharmaceutical Industries with distribution permit number DTL7226303037A1, box packaging, plastic bottle @ 60 ml.
- Unibebi Fever Syrup (fever medicine), produced by Universal Pharmaceutical Industries with distribution license number DBL8726301237A1, in box packaging, bottle @ 60 ml.
- Unibebi Fever Drops (fever medicine), produced by Universal Pharmaceutical Industries with distribution license number DBL1926303336A1, box packaging, bottle @ 15 ml.